What are nanobubbles?
Nanobubbles are extremely small gas bubbles. They have unique physical properties that make them very different from normal bubbles.

Neutral Buoyancy
Nanobubbles have a typical mean diameter of 100-300 nanometer. Ordinary bubbles quickly rise to the surface and burst; smaller nanobubbles have a low buoyancy and will remain suspended in liquids for an extended period of time until they dissolve. Due to Brownian motion they travel randomly through the water. This unique behavior enables nanobubbles to provide a homogeneous distribution of gas throughout the water.

Negative Surface Charge
All bubbles naturally possess a negative surface charge. The smaller the bubble, the stronger the surface charge. Nanobubbles have a high zeta potential, which is the electro kinetic potential in colloidal dispersions. The strong negative charge of nanobubbles limits their coalescence, therefore the integrity of the bubbles is preserved at any depth for extended periods of time.
Gas Reserve
The low buoyancy and negative surface charge of nanobubbles allow them to remain in suspension for a long time. This occurs even at concentrations much higher than the normal gas saturation. In this capacity, the nanobubbles act as a gas reserve in the solution. As gas is consumed from the water by biology, chemistry, or off-gassing, the nanobubbles rapidly diffuse more gas into the water, maintaining elevated dissolved gas levels until the nanobubbles are depleted. This additional gas reserve enables us to utilize gases more effectively than ever before.
Characterization of nanobubbles

Laser light
Randomly moving nanobubbles visible in green laser light

Moving bubbles
Moving oxygen nanobubbles in pure water, visualized by NanoTracking Analysis (NTA), Nanosight, Malvern.

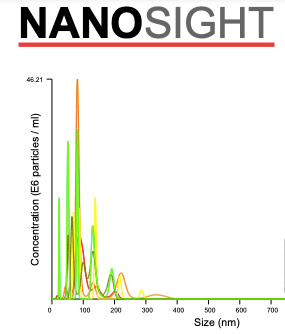
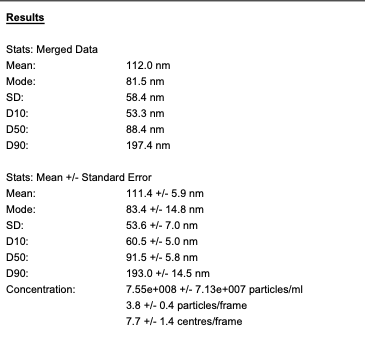
Bubble size
The average size of our nanobubbles is 110 nm, measured with Nanosight NTA.

Bubble concentration
And at DO of 280%, the measured concentration of the nanobubbles is 7x10^8/ml
Oxygen nanobubbles
Our nanobubble generators require little oxygen to dissolve large amounts of oxygen in water.
The nanobubble method is much more effective than blowing pure oxygen in water.

Blowing pure oxygen
Pure oxygen forms bubbles that rapidly rise to the surface and escape.

Oxygen nanobubbles
Nanobubbles do not coalescense and remain in the water.
Water oxygenation

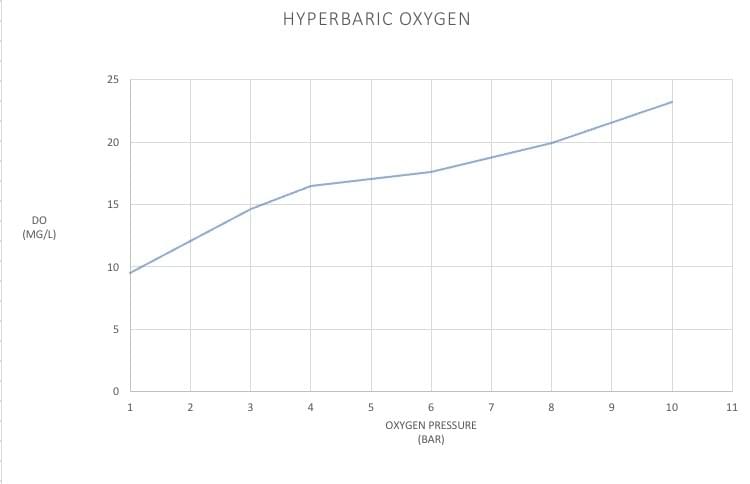
Dissolved oxygen (DO)
At room temperature and sea-level (1 bar), 8.68 mg/L oxygen can be dissolved in water (100% saturation level). Higher dissolved oxygen (DO) levels can be obtained by pushing compressed oxygen into the water (hyperbaric conditions). This is an inefficient process, requiring large amounts of oxygen gas.

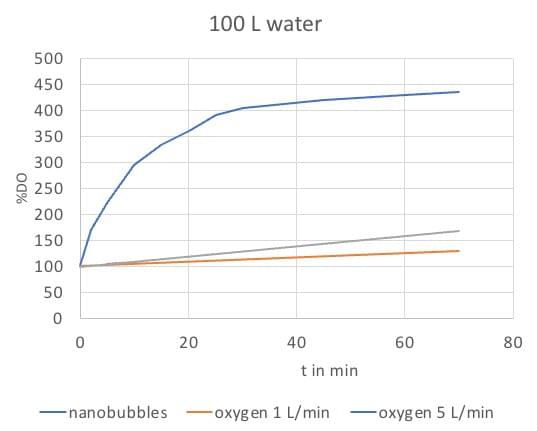
Nanobubble generator
Our nanobubble generator requires very little oxygen to dissolve increased amounts of oxygen in water, at only 1 bar of gas pressure. We use the hydrodynamic cavitation method to produce the nanobubbles, their concentration depends on circulation time in the machine. This method is much more effective than flushing water with pure oxygen, as is shown in this experiment with 100 L circulating water.
- After flushing water with 1 L/min and 5 L/min pure oxygen for 30 minutes, DO-level is increased to max. 130%.
- With use of the nanobubble generator (oxygen flow 1 L/min) DO-levels increase rapidly to 400 % in 30 min.

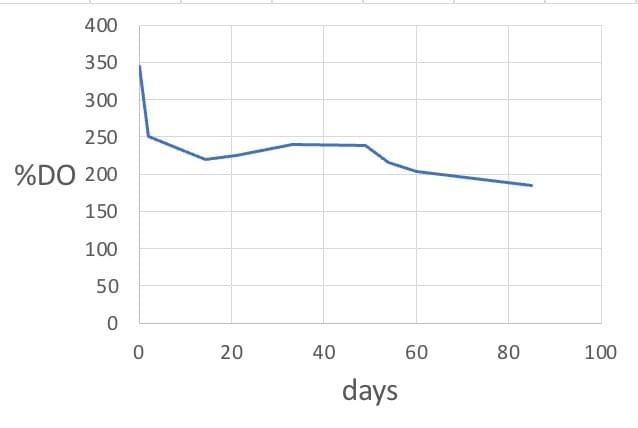
Persistent oxygen concentration
The excess oxygen gradually disappears from the water in 1 week.
In closed bottles, however, the high oxygen level in the water produced by the oxygen nanobubble generator persists for at least 3 months. The initial drop in DO is caused by evaporation of free oxygen upon opening the bottle. The remaining plateau level is maintained by the nanobubble-gas reserve.
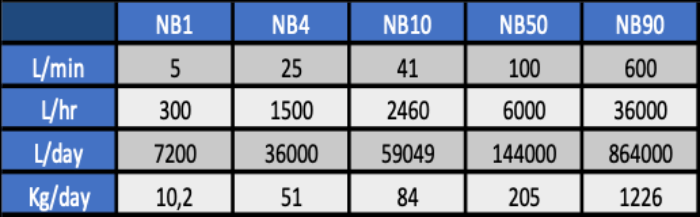
Capacity of our nanobubble generators
Transfer efficiency of our sytems for dissolving oxygen from ambient air in water is approx. 90%